Advanced molecular biology methods
While older ideas may remain timely, their applications change drastically. Even though no one may prepare a lambda library anymore, libraries are at the crux of next-generation sequencing. In this module, students will travel from Cot analysis to microarrays; from plasmids to NGS libraries; from restriction enzymes to Gateway cloning to Gibson and Goldengate assembly; and from PCR-RFLP and qPCR to allele-specific, isothermal, HRM, asymmetric, digital, inverse, ligation, methylation-specific and etc-PCR.
Instructor: Kostas Mathiopoulos
Dot blot and microarrays
The basic principles of dot blot for protein detection in a given sample, and comparison with other protein detection methods based on blotting, are presented. Adaptations of the method for antibody selection and screening approaches are included. In line with screening approaches, the students are introduced to the principles and design of microarrays, fluorescent labeling, and analysis of arrays of nucleic acid fragments attached to a solid surface (chip).
Instructor: Nikos Balatsos
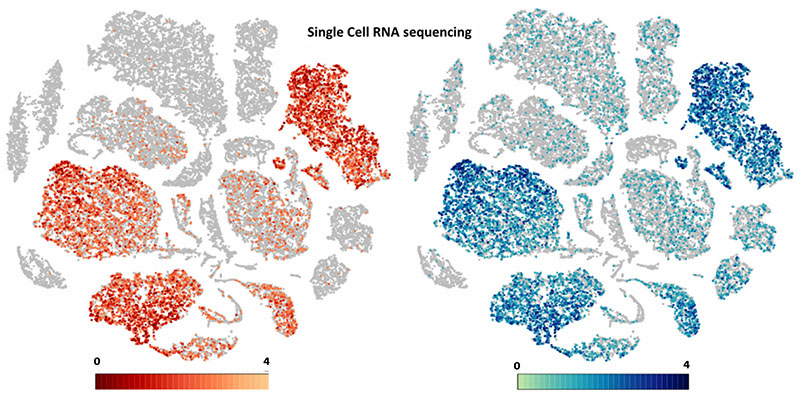
Advanced NGS applications
Next-generation sequencing has revolutionized all fields of modern biology. Current deep sequencing approaches have evolved beyond mere deciphering of nucleotide composition, providing insights into genome structure, evolution, function, and architecture. During the advanced NGS applications module, students are expected to present an experimental design that addresses a scientific question of their choice with the use of modern NGS platform applications. They are encouraged to focus on advanced NGS approaches, including but not limited to chromatin architecture, RNA-protein interactions, identification of DNA damage sites, single-cell expression, and short vs long read applications.
Instructor: Antonis Giakountis
Methods in gene functional analysis Ι: RNA interference (mammalian tissue culture)
Certain short RNA molecules have the capability to bind to endogenous mRNAs triggering their degradation, thus silencing gene expression. Stemming from this, researchers have developed various technologies for the analysis of gene functions. During this module, the principles of the method are presented, including complementarity and sequence design, advantages and challenges, as well as efficiency and adaptations for screening formats.
Instructor: Nikos Balatsos
Methods in gene functional analysis ΙI: CRISPR/Cas9
Since its functional elucidation in 2008, CRISPR/Cas9 has become the most rapidly expanding tool that transformed biology. Used either as a biotechnological product, an evolutionary paradox, an agricultural engineering toolbox, or a gene therapy asset, CRISPR/Cas never seizes to progress scientific creativity. During this module, students are introduced to cutting-edge applications of the CRISPR/Cas universe. Students are expected to address a specific scientific question regarding gene function using at least one CRISPR/Cas application in their organism of choice. Feasibility of application, selection of appropriate controls, caveats, and contingency measures are all taken into account for justifying their experimental design.
Instructor: Antonis Giakountis
Methods in gene functional analysis ΙII: Cre recombinase
During this module, students learn the utility of genetically engineered mouse models in biomedical research and the underlying technology. A brief presentation of the types of genetic modifications (insertion, knockout, conditional mutant) focusing mainly on the strategy followed by the European Conditional Mouse Mutagenesis is initially presented, followed by discussions aiming at the deeper exploration of the course content, questions & answers as well as problem-solving.
Instructor: Theologia Sarafidou
Methods in gene functional analysis ΙV: Transgenic plants
This module covers the methodology used in Plant Science, focusing on plant molecular biology and plant biotechnology. An introduction is given by the instructors on classical, novel, and groundbreaking methods used by scientists in our days to yield new knowledge and solve novel problems. Students are asked to design research on a given topic and describe in detail the methodology they will follow to: a) identify novel genes, b) isolate the genes and c) verify the responsible genes for a given phenotype.
Instructors: Kalliope Papadopoulou & Daniela Tsikou
Methods in gene functional analysis ΙV: Transgenic insects
All animals are equal, but Drosophila melanogaster is the most equal of all! Practically all modern technologies have been tried, if not developed, in this organism. And, consequently, this has led to unimaginable applications in insect engineering for practical purposes, such as moths that produce spider silk for bulletproof vests, flies that produce male-only progeny to control fly populations, mosquitoes that are incapable of spreading malaria.
Instructor: Kostas Mathiopoulos
Model species in biological research
Model organisms that are extensively studied to understand biological phenomena are briefly introduced. Aspects of their conserved genetic elements that govern metabolic and developmental pathways as well as their uniqueness that renders them better subjects for certain research areas are covered. During this module, students analyze how a specific scientific question is studied in model organisms. Students are encouraged to review comparatively how different model organisms contributed to disentangling a particular scientific question based on their evolutionary history, genomic structure, genetic resources, and ecological function.
Instructors: Katerina Moutou & Kalliope Papadopoulou