Systems and methods for heterologous expression of proteins
Comprehensive presentation of the modern systems (prokaryotic, eukaryotic, and in vitro systems) for heterologous expression of proteins. Use of case studies as examples for the thorough discussion of the applied methodologies using the E.coli system with emphasis on technical issues such as choice of expression vectors, cloning strategies, choice of expression cell lines, expression tests, and optimization of expression. Presentation of the methodologies that should be followed in order to ensure the structural and functional integrity of the expressed proteins (sample preparation). The students will be able to design a detailed protein expression protocol.
Instructor: Vicky Skamnaki
Protein purification methods, liquid chromatography techniques
Overview of protein purification with emphasis on liquid chromatography techniques. The lecture focuses on issues such as the choice of purification tags, the use of automated FPLC systems, the analysis of chromatograms, the strategies in the choice, and the steps of the purification methods. The students will be able to design detailed protein purification strategies.
Instructor: Vicky Skamnaki
Proteomics analysis I. Protein identification with a combination of liquid chromatography and mass spectroscopy, proteomes comparison
Current methodologies and trends in the field of proteomics are presented. Students will be introduced to the logic and methodology underpinning sample preparation, complex mixture fractionation, protein separations, and the state-of-the-art techniques used in proteomics. They should be able to describe and discuss the possibilities and advantages of various proteomics technologies and compare traditional methods with emerging technologies.
Instructor: Maria Kontou
Proteomicsanalysis II. Applications: Determination of protein modifications, protein-protein interaction
Different proteomics applications used today are thoroughly presented, from experimental planning to data analysis. Students should be able to develop the ability to suggest suitable approaches for different scientific problems and have a general understanding of how proteomics fits into the broader picture of systems biology and biological and clinical research.
Instructor: Maria Kontou
Protein-nucleic acid interactions
Technologies for the study of an organism’s transcriptome at a given time or under certain conditions are presented. Nuclease protection assay principles are used to understand transcriptomics’ principles, including RNA extraction, design of probes and hybridization, treatment with RNases to cleave single-stranded RNA, and determination of sequences of interest. In addition, the systematic evolution of ligands by exponential enrichment (SELEX) is presented, a method used to isolate specific single-stranded RNA or DNA sequences (aptamers) that interact with a ligand (e.g., a DNA binding protein of choice).
Instructor: Nikos Balatsos
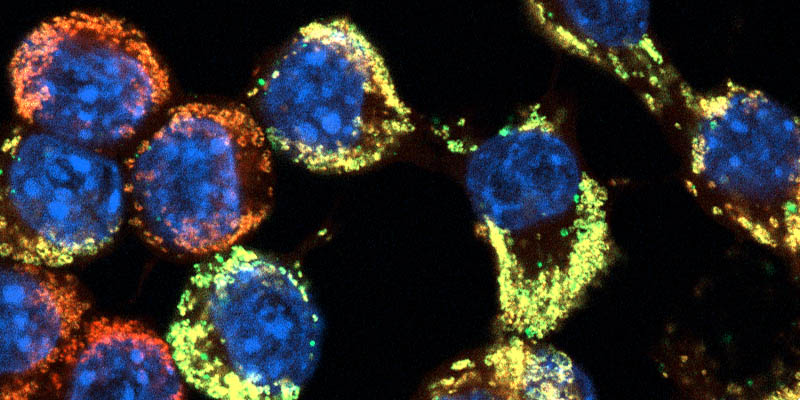
Confocal microscopy – Applications
Students will be introduced to theoretical and practical knowledge on confocal microscopy principles and applications. Basic and advanced confocal microscopy applications, such as single section, time and z-series acquisition, 3D reconstruction, colocalization studies, FRAP, FRET, FLIP analysis are presented. Students practiced designing experimental protocols on confocal microscopy analysis.
Instructor: Anna-Maria Psarra
Protein immunochemical methods
Students are provided with theoretical and practical knowledge on various immunochemical analysis methods including Western blot analysis, immunohistochemistry, immunocytochemistry, immunoprecipitation, ELISA, and proximity ligation assay. Students will practice designing experimental protocols on immunochemical analysis methods.
Instructor: Anna-Maria Psarra
Biophysical methods for protein analysis. Protein NMR spectroscopy and X-ray crystallography
Students are presented with theoretical and practical knowledge sufficient to use a variety of biophysical methods on biochemical systems. An understanding of the physical principles underpinning the techniques is provided, as well as an extensive description of the technologies and their applications in biochemical analysis. Emphasis is given to Macromolecular X-ray crystallography, NMR spectroscopy, and cryo-electron microscopy.
Instructor: Demetres Leonidas
Flow cytometry
Basic principles and terms of flow cytometry are presented. All the parts of a flow cytometer and their role for samples’ analysis are described. Moreover, the different methods and diagrams used for the presentation and analysis of the results are explained. In addition, various applications and protocols of flow cytometry are presented. Thus, at the end of the course, the student will be able to understand how a flow cytometer works, its basic applications and the analysis of the results.
Instructor: Dimitrios Stagos
Antioxidant activity analysis
Antioxidant status in the cell is governed by complex molecular and biochemical events. The redox status is divided into two main pillars. The antioxidants machinery and the damage events to biological molecules by free radicals. In this short module, students experience in the lab how both these pillars are analyzed and assessed (measurement of total antioxidant state by DPPH, ABTS, OH radical and superoxide radical assessment, and also glutathione, protein oxidation, lipid hyperoxidation and catalase).
Instructor: Dimitrios Kouretas
Kinetic analysis of applied enzymatic reactions and fermentations
In the food and drink industry, the determination of kinetic parameters of enzymatic reactions and fermentations is useful for the quality control of traditional products, as well as for the creation of novel foods. Methods to determine kinetic parameters are presented in specific food products.
Instructor: Persephoni Giannouli